Following the completion of trials that found its efficacy at 56 percent, the first safe and effective dengue vaccine in the world could be out commercially in the country by July next year.
Produced by French pharmaceutical giant Sanofi Pasteur, the vaccine offers varying degrees of protection against each of the four known types of dengue virus: 73 percent effective for Den-3 and Den-4; 50 percent for Den-1; and 35 percent for Den-2. With these findings recently published online in The Lancet medical journal, the company expects to reduce dengue hemorrhagic fever cases by 88.5 percent.
“To put this into perspective, our vaccine—when licensed and approved with the appropriate coverage—has the potential to halve the 50 to 100 million estimated cases of infections a year, and nearly eliminate the estimated 500,000 cases that will develop the severe form of the disease,” said Nicholas Jackson, Sanofi Pasteur’s dengue vaccine research head during a presentation held at Sofitel Philippine Plaza Manila in Pasay City.
1.8B in Asia
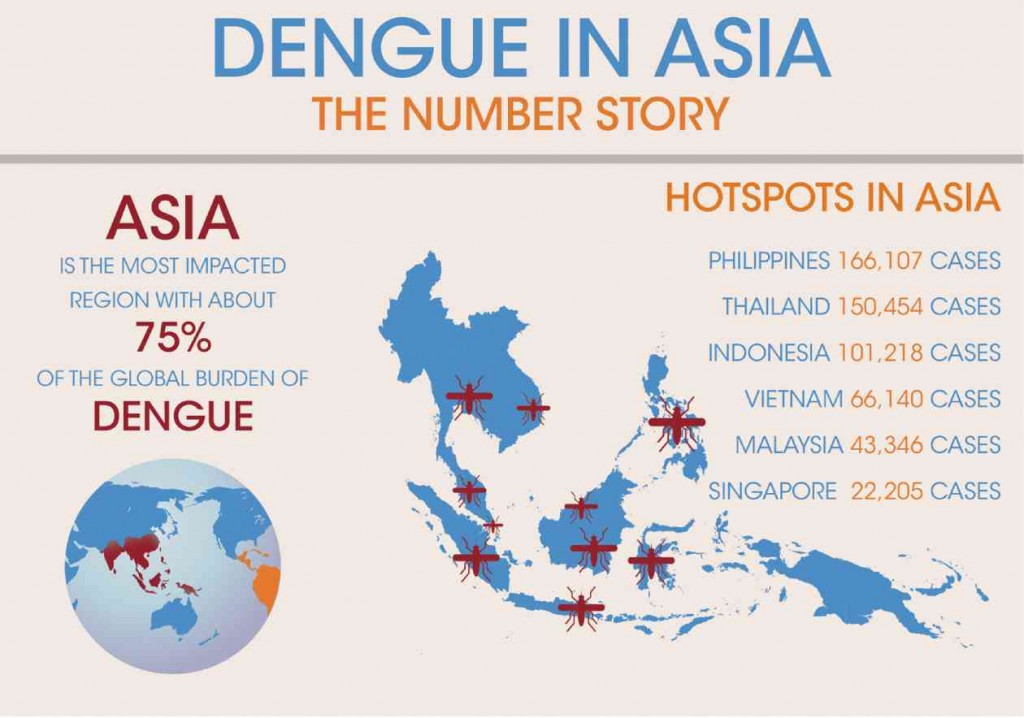
It is estimated that among the 2.5 billion people at risk globally from dengue infection, about 1.8 billion of them are in Asia. In fact, over the past 50 years, the incidence of dengue has increased by 30-fold, with Asia as the most impacted region, representing approximately 75 percent of the global burden of dengue.
Health Secretary Enrique Ona said the vaccine, once approved, should significantly contribute to achieving the World Health Organization’s global strategy for dengue prevention and control by reducing mortality by 50 percent and reducing morbidity by 25 percent by 2020.
In Asia, the Philippines is among the most affected, with an estimated 166,107 cases reported annually. Moreover, dengue infection is the leading cause of childhood hospitalizations, 36 percent of which afflicts Filipinos between one to nine years old.
Although the 31,088 cases reported as of last July 2 were a big decrease from last year’s 79,274 cases (dengue-related deaths also decreased from 307 to 134), the Department of Health warned that the number of dengue cases is expected to increase in the next few months due to the rainy season.
This is why the Philippines was included in the trial vaccine’s test sites, namely, Cebu City and San Pablo City in Laguna. Other Southeast Asian test sites were Kamphaeng Phet and Ratchaburi in Thailand, Kuala Lumpur and Penang in Malaysia, Bali and West Java in Indonesia, and Long Xuen City and My Tho City in Vietnam.
10,275
According to Dr. Rosario Capeding, head of the dengue research program of the DOH’s Research Institute for Tropical Medicine, a total of 10,275 healthy kids were enrolled in the late-stage (Phase 3) study of Sanofi Pasteur’s candidate vaccine known as CYD (chimeric yellow fever) dengue vaccine, 3,500 of which come from the Philippines.
She also added that the vaccine was found to be safe with no serious side effects.
The children—between two to 14 years old—who participated in the trial from June 2011 to June 2013 received three 0.5-millimeter injections of the CYD dengue vaccine at six-month intervals.
Capeding related that it was not easy to formulate a dengue vaccine because the disease, primarily spread by the Aedes aegypti mosquito, had four serotypes and that no animals had been found suitable for testing (the animal subjects were somehow able to develop a natural biochemical defense mechanism against the disease).
Over 20 years
In developing the vaccine, Sanofi Pasteur invested more than $1.77 billion in intensive research for over 20 years—several years ahead of potential competitors. In fact, the company even built a dedicated factory near Lyon in southern France with a capacity to produce 100 million doses a year.
According to Sanofi Pasteur dengue vaccine head Guillaume Leroy, the company intends to make the vaccine available first in countries suffering the most from dengue. Leroy added that the price would only be determined after discussions with the WHO and the countries interested in purchasing the vaccine.
In the country, Leroy said Sanofi Pasteur will provide the DOH all necessary data about the vaccine and apply for a license.